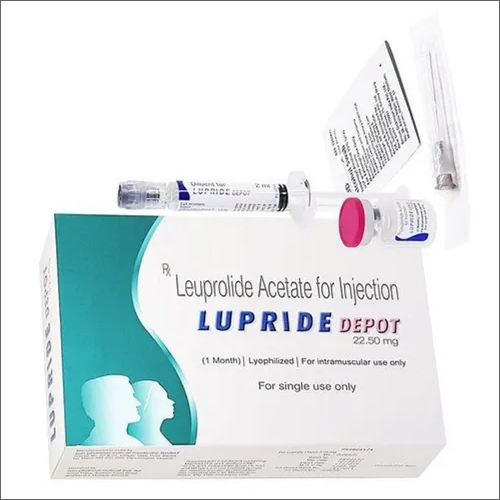
Lupride depot Injection
15000 INR/Box
Product Details:
- Salt Composition Leuprolide Acetate
- Indication Prostate cancer, endometriosis, uterine fibroids, precocious puberty
- Dosage Form Injection
- Enzyme Types Other
- Feature Depot formulation for sustained release
- Ingredients Leuprolide Acetate
- Application Hormonal therapy
- Click to View more
X
Lupride depot Injection Price And Quantity
- 1 Box
- 15000 INR/Box
- 3.75 mg/vial or 11.25 mg/vial
- Yes
- Intramuscular injection
- Single-dose vial with sterile solvent for reconstitution
- Lupride Depot
- Schedule H (Prescription Drug)
Lupride depot Injection Product Specifications
- Leuprolide Acetate
- White to off-white lyophilized powder
- Leuprolide Acetate
- Hormonal therapy
- Depot formulation for sustained release
- Other
- Dry place
- Prostate cancer, endometriosis, uterine fibroids, precocious puberty
- Slight Smell
- 24 months
- Injection
- 3.75 mg/vial or 11.25 mg/vial
- Yes
- Intramuscular injection
- Single-dose vial with sterile solvent for reconstitution
- Lupride Depot
- Schedule H (Prescription Drug)
Product Description
Technical Specification
- Country of Origin : Made in India
- Form : Injection
- Brand : Lupride Deport
- Prescription/Non prescription : Prescription
- Composition : Leuprolide Acetate for injection
Sustained-Release Hormonal Therapy
Lupride Depot Injection offers the advantage of depot formulation, ensuring a prolonged release of medication for effective hormonal management. This helps minimize the need for frequent dosing, improving patient compliance and facilitating optimal treatment of hormone-responsive conditions like prostate cancer and endometriosis.
Versatile Indications for Use
This injection is approved for a range of indications, including prostate cancer, endometriosis, uterine fibroids, and precocious puberty. The reliable delivery and sustained-release mechanism make it a preferred choice for both adult and pediatric patients requiring hormonal modulation therapy.
FAQ's of Lupride depot Injection:
Q: How should Lupride Depot Injection be administered?
A: Lupride Depot Injection is administered as an intramuscular injection by a healthcare professional. The lyophilized powder should be reconstituted with the provided sterile solvent before injection, following the preparation guidelines included with the product.Q: What conditions is Lupride Depot Injection used to treat?
A: Lupride Depot Injection is indicated for the treatment of advanced prostate cancer, endometriosis, uterine fibroids, and precocious puberty. It modulates hormone production to manage these hormone-sensitive conditions effectively.Q: When will the effects of Lupride Depot begin to work, and how long do they last?
A: The sustained-release formulation of Lupride Depot allows for gradual hormone regulation, with effects typically becoming noticeable within a few days to weeks after administration. The depot effect maintains therapeutic drug levels, lasting for several weeks per injection depending on the prescribed dose.Q: Where should Lupride Depot Injection be stored before use?
A: Store Lupride Depot Injection in a dry place at the recommended temperature as indicated in the package insert. Avoid exposure to moisture or excessive heat to maintain stability and shelf life.Q: What are the benefits of the depot formulation in Lupride Injection?
A: The depot formulation ensures a sustained release of medication, reducing the need for frequent injections. This provides consistent therapeutic effects and enhances patient convenience and adherence to treatment protocols.Q: Who can prescribe Lupride Depot Injection?
A: Lupride Depot is a Schedule H prescription drug, which means it can only be prescribed by a licensed medical practitioner qualified to diagnose and treat hormone-related disorders.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email