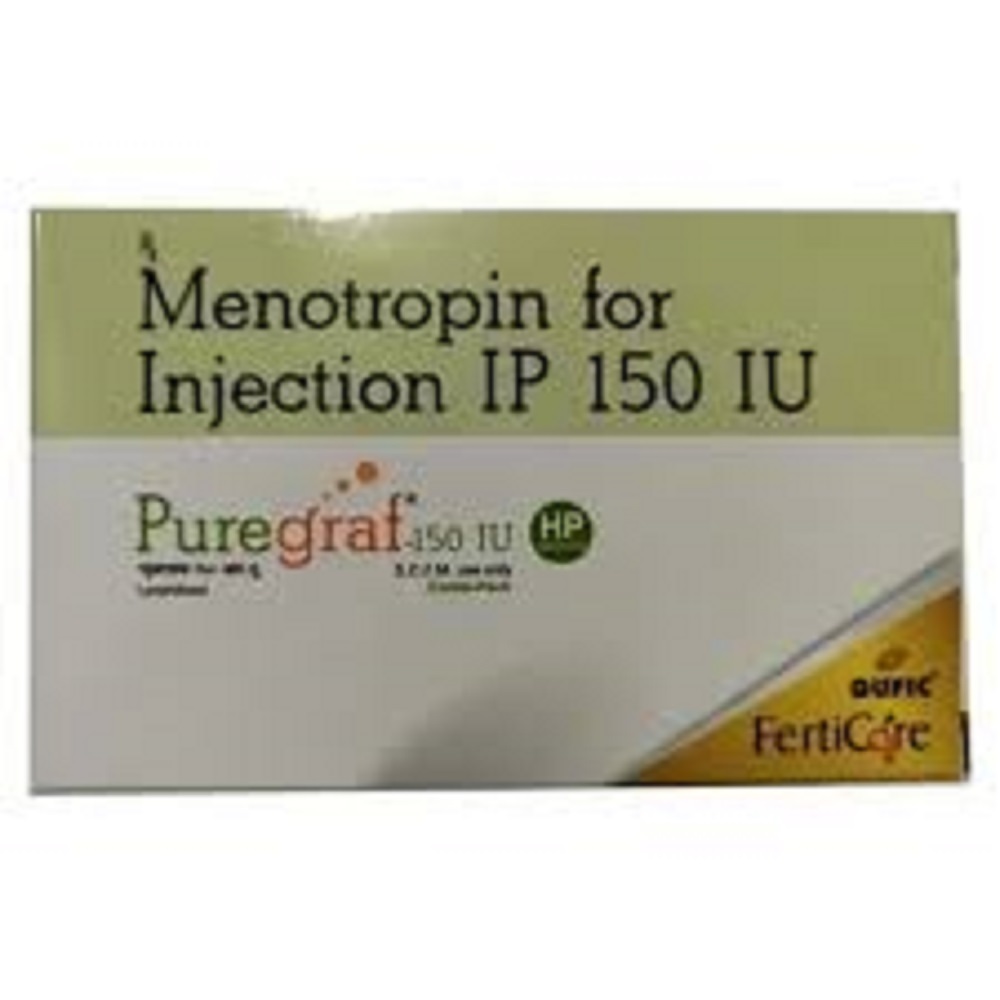
Puregraf 150 Iu Hp Injection
950 INR/Piece
Product Details:
- Indication Infertility, Ovulation induction, Assisted Reproductive Technology (ART) procedures
- Dosage Form Lyophilized powder for injection
- Pacakaging (Quantity Per Box) 1 Vial and solvent ampoule per box
- Origin of Medicine Biotechnological
- Salt Composition Menotrophin (Human Menopausal Gonadotrophin, HP-HMG) 150 IU
- Drug Type Hormonal gonadotropin
- Ingredients Highly Purified Human Menotrophin (HP-HMG)
- Click to View more
X
Puregraf 150 Iu Hp Injection Price And Quantity
- 1 Piece
- 950 INR/Piece
Puregraf 150 Iu Hp Injection Product Specifications
- Store in a refrigerator (2C 8C), do not freeze, protect from light
- Hormonal gonadotropin
- Lyophilized powder for injection
- Infertility, Ovulation induction, Assisted Reproductive Technology (ART) procedures
- As prescribed by the physician, typically 150 IU per administration
- Administer subcutaneously or intramuscularly as per medical advice
- Biotechnological
- Female infertility treatment, Assisted reproductive techniques
- Women (adults) undergoing ovulation induction
- 1 vial per box
- Injection
- Highly Purified Human Menotrophin (HP-HMG)
- Stimulates ovarian follicle growth and ovulation
- 1 Vial and solvent ampoule per box
- Menotrophin (Human Menopausal Gonadotrophin, HP-HMG) 150 IU
Product Description
Puregraf 150 IU HP Injection is a remarked, resplendent option specially crafted for women seeking advanced intervention in infertility treatments. This grandiose hormonal gonadotropin, containing highly purified Human Menotrophin (HP-HMG), is indicated for ovulation induction and assisted reproductive technology (ART) procedures. Each select box includes a lyophilized powder vial and solvent, designed for subcutaneous or intramuscular administration following reconstitution. This registered pharmaceutical product is only to be used under specialist supervision, with storage between 2C-8C. Experience the assurance of quality and efficacy for your reproductive journey.
Resplendent Solution for Female Infertility: Specific Usage and Site of Application
Puregraf 150 IU HP Injection is specially indicated for women undergoing ovulation induction as part of infertility treatment or Assisted Reproductive Techniques (ART). Administered subcutaneously or intramuscularly, this select formulation harnesses the power of highly purified HP-HMG to stimulate ovarian follicle growth and ovulation. Used strictly as prescribed by physicians in hospital or specialist settings, it provides targeted support in reproductive care for women seeking advanced conception assistance.
Advance Purchase: Sample Policy, Certifications, and Packaging Details
Puregraf 150 IU HP Injection is shipped promptly after confirmed quotation acceptance, ensuring timely delivery for medical needs. Each package consists of one vial of lyophilized powder and a solvent ampoule, securely packed to ensure stability. Dispatched with all relevant certifications and quality documents, this registered hormonal preparation complies with pharmaceutical standards. Whether you are a dealer, exporter, or hospital pharmacy, expect reliable packaging and traceability with every grandiose Puregraf shipment.
Resplendent Solution for Female Infertility: Specific Usage and Site of Application
Puregraf 150 IU HP Injection is specially indicated for women undergoing ovulation induction as part of infertility treatment or Assisted Reproductive Techniques (ART). Administered subcutaneously or intramuscularly, this select formulation harnesses the power of highly purified HP-HMG to stimulate ovarian follicle growth and ovulation. Used strictly as prescribed by physicians in hospital or specialist settings, it provides targeted support in reproductive care for women seeking advanced conception assistance.
Advance Purchase: Sample Policy, Certifications, and Packaging Details
Puregraf 150 IU HP Injection is shipped promptly after confirmed quotation acceptance, ensuring timely delivery for medical needs. Each package consists of one vial of lyophilized powder and a solvent ampoule, securely packed to ensure stability. Dispatched with all relevant certifications and quality documents, this registered hormonal preparation complies with pharmaceutical standards. Whether you are a dealer, exporter, or hospital pharmacy, expect reliable packaging and traceability with every grandiose Puregraf shipment.
FAQ's of Puregraf 150 Iu Hp Injection:
Q: How should Puregraf 150 IU HP Injection be prepared for administration?
A: Puregraf 150 IU HP Injection requires reconstitution before use. The lyophilized powder should be mixed with the provided solvent to prepare the injection, following the instructions given by healthcare professionals.Q: What are the main uses of Puregraf 150 IU HP Injection?
A: This injection is chiefly used for the treatment of female infertility, particularly for ovulation induction and assisted reproductive technologies (ART), by stimulating ovarian follicle growth.Q: When is Puregraf 150 IU HP Injection contraindicated?
A: Puregraf should not be used in cases of hypersensitivity to HMG, ovarian or primary pituitary failure, or unexplained uterine bleeding. Always consult your physician for assessment before use.Q: Where should Puregraf 150 IU HP Injection be stored?
A: Store the injection in a refrigerator at 2C-8C. Keep it protected from light and do not freeze it.Q: What special precautions should be considered during treatment?
A: Ovarian response should be monitored closely to avoid complications like multiple pregnancies or ovarian hyperstimulation syndrome. Use is restricted to hospital or specialist supervision only.Q: What are the potential adverse reactions of Puregraf 150 IU HP Injection?
A: Common adverse effects include headache, abdominal pain, ovarian hyperstimulation syndrome (OHSS), and possible allergic reactions. Immediate medical consultation is advised if serious side effects occur.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email