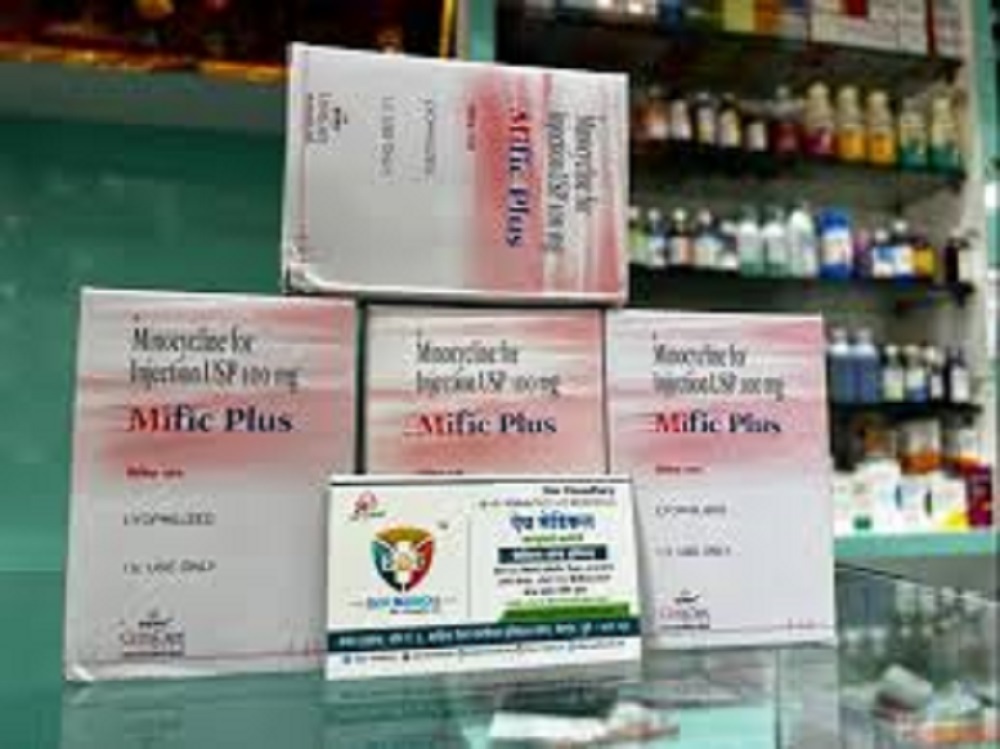
Mific plus 100 mg injection
Product Details:
- Salt Composition Mifepristone 100 mg
- Indication Termination of pregnancy up to 49 days of gestation
- Dosage Form Injection
- Drug Type General Medicines
- Ingredients Mifepristone IP
- Physical Form Liquid
- Recommended For Medical termination of intrauterine pregnancy
- Click to View more
Mific plus 100 mg injection Price And Quantity
- 700.0 INR/Piece
- 1 Piece
Mific plus 100 mg injection Product Specifications
- 1 vial Pieces
- 100 mg as prescribed by the physician
- Liquid
- Adults
- Mifepristone 100 mg
- Store in a cool, dry place below 25 C, protect from light
- Termination of pregnancy up to 49 days of gestation
- To be administered intramuscularly or as directed by physician
- Mifepristone IP
- General Medicines
- Injection
- Medical termination of intrauterine pregnancy
Mific plus 100 mg injection Trade Information
- 100 Piece Per Month
- 7 Days
Product Description
Mific Plus 100mg Injection may also be used to treat many sexually transmitted diseases. It helps to improve your symptoms and cure the underlying infection. It is given as a drip (intravenous infusion) into a vein under the supervision of a doctor or a nurse. You should get the injection regularly at evenly spaced intervals. Do not skip any doses and finish the full course of treatment even if you feel better. Stopping the medicine too early may lead to the infection returning or worsening.
The most common side effects of this medicine include headache, dizziness, nausea, vomiting, diarrhea, and skin reaction on exposure to sunlight (photosensitivity). Avoid excessive sun exposure and use sunscreen and protective clothing when outdoors. Some people may develop temporary redness or pain at the site of injection. These side effects are usually mild but let your doctor know if they bother you or last more than a few days.
Before using it, you should tell your doctor if you are allergic to any antibiotics or have any liver or kidney problems. You should also let your doctor know all other medicines you are taking as they may affect, or be affected by this medicine. Pregnant and breastfeeding mothers should consult their doctor before using it. It may blur your vision or make you feel sleepy and dizzy. Do not drive if these symptoms occur.
Medical Use and Efficacy
Mific Plus is specifically designed for the medical termination of intrauterine pregnancies up to 49 days gestation. The inclusion of Mifepristone 100 mg IP ensures a targeted abortifacient action. It offers a non-surgical solution, providing a safe and controlled process under clinical care for eligible patients.
Administration and Dosage
This medication is given through intramuscular injection, following strict guidelines provided by a healthcare practitioner. Dosage recommendations (100 mg) are tailored by the treating physician for maximal efficacy and patient safety, ensuring individualized care during the procedure.
Safety and Regulatory Approval
Mific Plus is approved by the Central Drugs Standard Control Organization (CDSCO), affirming its safety and quality standards for use in India. It is essential to follow prescribed usage and consult health professionals to avoid contraindications and minimize potential side effects.
FAQ's of Mific plus 100 mg injection:
Q: How is Mific Plus 100 mg injection administered?
A: Mific Plus is administered as an intramuscular injection by a healthcare professional, ensuring precise dosing and safety during the medical termination process.Q: What are the benefits of using Mific Plus for pregnancy termination?
A: It provides a safe, effective, and non-surgical option for terminating intrauterine pregnancy up to 49 days of gestation under medical supervision.Q: When should Mific Plus injection be used?
A: This medication is recommended for adults seeking medical termination of intrauterine pregnancy within 49 days of gestation, as determined by their physician.Q: Where should Mific Plus vials be stored?
A: Store the product in a cool, dry place below 25C, protected from light, as per packaging instructions to maintain efficacy and safety.Q: What precautions and contraindications should be considered before using Mific Plus?
A: It should not be used if you have known hypersensitivity to Mifepristone, chronic adrenal failure, hemorrhagic disorders, or inherited porphyrias. Always consult your doctor before use.Q: What are the possible side effects of this injection?
A: Possible side effects include abdominal pain, nausea, vomiting, and vaginal bleeding. Notify your healthcare provider if you experience any adverse symptoms.Q: Is a prescription required to obtain Mific Plus?
A: Yes, a valid medical prescription is required for purchase and administration of Mific Plus in India.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+