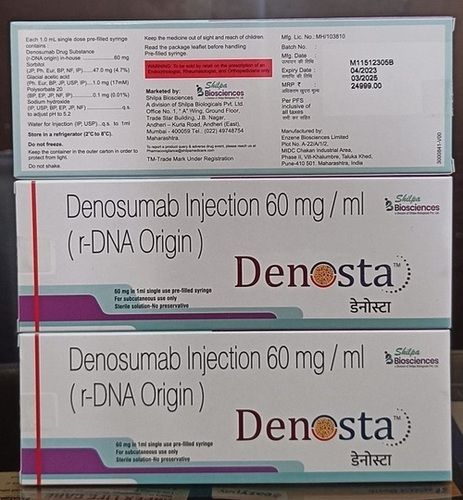
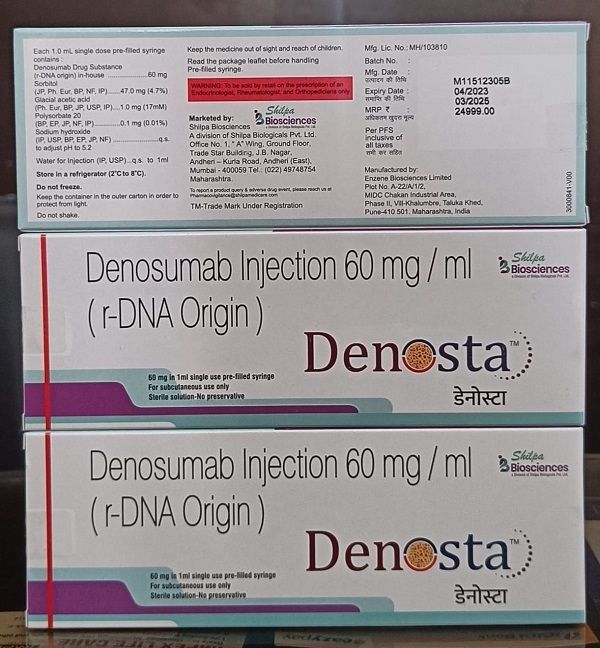
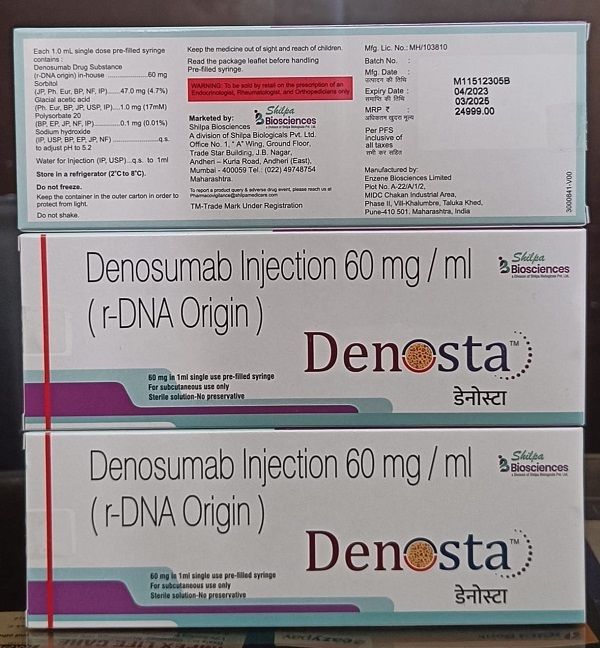
Denosta 60 Mg Injection
Product Details:
- Indication Treatment of osteoporosis and bone loss due to various causes including hormone deprivation in cancer
- Pacakaging (Quantity Per Box) 1 prefilled syringe per box
- Salt Composition Denosumab
- Origin of Medicine Biotechnology-derived (biologic) medicine
- Dosage Form Injection (solution for injection)
- Drug Type Monoclonal Antibody
- Ingredients Denosumab 60 mg
- Click to View more
Denosta 60 Mg Injection Price And Quantity
- 6500 INR/Pack
- 1 Pack
- Clear to slightly opalescent, colorless to pale yellow solution
- Monitor calcium levels prior to starting therapy and during treatment
- Musculoskeletal pain, urinary tract infection, upper respiratory tract infection, sciatica, rash
- Subcutaneous injection
- Prescription
- Denosta
- Antiosteoporotic
- Hypocalcemia, hypersensitivity to denosumab
- 24 months from manufacturing date
- 60 mg/ml
Denosta 60 Mg Injection Product Specifications
- Reduces bone loss, increases bone mass and strength
- 1 prefilled syringe
- Osteoporosis and bone loss treatment
- As prescribed by physician
- Injection
- Store in a refrigerator (2C 8C); Do not freeze
- Treatment of osteoporosis and bone loss due to various causes including hormone deprivation in cancer
- Administered as a subcutaneous injection every 6 months
- Denosumab 60 mg
- Denosumab
- Biotechnology-derived (biologic) medicine
- Adults
- Injection (solution for injection)
- 1 prefilled syringe per box
- Monoclonal Antibody
- Clear to slightly opalescent, colorless to pale yellow solution
- Monitor calcium levels prior to starting therapy and during treatment
- Musculoskeletal pain, urinary tract infection, upper respiratory tract infection, sciatica, rash
- Subcutaneous injection
- Prescription
- Denosta
- Antiosteoporotic
- Hypocalcemia, hypersensitivity to denosumab
- 24 months from manufacturing date
- 60 mg/ml
Product Description
Introduction to Denosta 60mg Injection
Denosta 60mg injection consists of the active ingredient Denosumab. It is a medication used to treat osteoporosis, characterized by weakened bones. It is also prescribed to prevent bone complications in patients with certain types of cancer, such as multiple myeloma and bone metastases. Denosta 60mg injection works by inhibiting a protein called RANK ligand, which plays a role in the breakdown of bone tissue. Studies proved it can significantly increase bone density, reducing fracture risk in postmenopausal women with osteoporosis and patients with cancer-related bone loss. Close monitoring of renal function may be necessary for patients with moderate renal impairment.
Denosta 60mg injection should not be used in individuals with known hypersensitivity or allergic reactions to its components. Adequate calcium and vitamin D supplementation is required to ensure during the treatment. It is not approved for use in children and adolescents. Its safety and effectiveness in pediatric populations have not been established, so its use is contraindicated in individuals under 18. This medication should be used cautiously in individuals with pre-existing hypoparathyroidism, characterized by low parathyroid hormone levels.
Mechanism of Action
Denosta 60 Mg Injection features denosumab, a monoclonal antibody that inhibits the activity of RANKL, a protein responsible for bone breakdown. By blocking RANKL, Denosta slows bone loss and helps increase bone density, providing effective management for osteoporosis and bone loss due to cancer therapies.
Administration and Usage
Administer Denosta as a subcutaneous injection every six months, using the prefilled syringe provided. The injection should be given by a healthcare professional, after confirming normal calcium levels. Ensure that the solution remains clear to pale yellow, and do not use if discolored. Always follow storage instructions and keep the product refrigerated.
Safety and Monitoring
Prior to beginning Denosta therapy, monitor calcium levels, as the medication is contraindicated in those with hypocalcemia or hypersensitivity to denosumab. Common side effects include muscle pain, urinary tract infections, upper respiratory infections, and rash. Patients should report any adverse effects promptly to their physician.
FAQ's of Denosta 60 Mg Injection:
Q: How is Denosta 60 Mg Injection administered?
A: Denosta 60 Mg Injection is administered by subcutaneous injection every six months, typically by a healthcare professional. The prefilled syringe provides a single dose for adult patients, and the injection should be given after confirming normal calcium levels.Q: What conditions is Denosta 60 Mg Injection recommended for?
A: Denosta is prescribed for the treatment of osteoporosis and bone loss associated with hormone deprivation in cancer, as well as other conditions leading to reduced bone mass. It helps in both preventing fractures and improving bone strength.Q: When should Denosta 60 Mg Injection not be used?
A: Denosta should not be used in individuals with hypocalcemia or known hypersensitivity to denosumab. It is essential that any contraindications are discussed with your healthcare provider before starting treatment.Q: Where should Denosta 60 Mg Injection be stored?
A: Store Denosta injection in a refrigerator at a temperature between 2C and 8C. Do not freeze the product, and keep it in the original packaging until it is used to protect it from light.Q: What are the common side effects of Denosta 60 Mg Injection?
A: Common side effects include musculoskeletal pain, urinary tract infection, upper respiratory tract infection, sciatica, and rash. Notify your physician if you experience any unusual or persistent symptoms during treatment.Q: What benefits does Denosta 60 Mg Injection offer?
A: Denosta effectively reduces bone loss, increases bone mass and strength, and lowers the risk of fractures in patients suffering from osteoporosis or bone loss due to certain cancer treatments, thereby improving overall bone health.Q: Is monitoring required during Denosta therapy?
A: Yes, calcium levels should be monitored before starting Denosta and during treatment, especially in individuals at risk for low calcium. Regular follow-up with your healthcare provider ensures safety and effectiveness.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+